In January 1865 a paper, “On the constitution of aromatic substances”, from August Kekulé in Ghent was read out to the Paris Chemical Society. The paper described the structure of the benzene molecule as a ring of six carbon atoms with one hydrogen atom attached to each carbon atom. Who was Kekulé? Why was his paper important? What is benzene?
Benzene is a colourless liquid with a pleasant (aromatic) smell and a boiling point of 80oC. It is an important industrial chemical used in the manufacture of polystyrene and other plastics, dyes, drugs, antiseptics, explosives and detergents. It was often used as a solvent but was discovered to be toxic so its uses are strictly controlled today. It is obtained now from crude oil but until the 1950s was extracted from coal tar. Coal tar is one product of heating coal without air. The other products are coke, coal gas and an oily liquid. The process was developed in the early nineteenth century to provide coal gas for lighting in streets and buildings. It was the source of household gas in the UK until natural gas from the North Sea became available in the 1960s.
It was the use of gas for lighting that lead to the discovery of benzene in 1826. At the Royal Institution in London, Michael Faraday was investigating a gas made by heating fish and whale oils in a furnace. The gas produced was used as a portable fuel for lamps. When Faraday compressed the gas he obtained a colourless liquid which he found was composed of carbon and hydrogen. He called the substance dicarburetted hydrogen. That is, he thought that its formula was C2H. This was because at the time it was thought that the atomic mass of carbon was 6 not 12. If we use the modern masses the simplest formula of benzene would work out to be CH. In 1834 Eilhardt Mitscherlich made a substance that he realised was the same as that discovered by Faraday but he gave it the name benzene. Soon after, the same substance was discovered in coal tar and it quickly became an important chemical commodity and it was found that its molecules actually contained six atoms each of carbon and hydrogen.
The structure of molecules was one of the chemical problems that became important in the mid-nineteenth century. Lavoisier’s work in the 1780s re-organised chemistry and gave chemists the ideas of elements and compounds that we have today. In 1805 John Dalton’s atomic theory provided a picture of elements made up of hard, indestructible balls that somehow could link together to form compounds.
Work by many different chemists on the composition of compounds lead Edward Frankland to suggest the theory of valency, or combining numbers, in 1852. Frankland’s theory suggested that the atoms of each element could form a certain number of links or “bonds” with other atoms. For example Hydrogen atoms (valency 1) could form 1 bond, oxygen atoms (valency 2) two bonds and carbon (valency 4) four bonds. This explained why the formula of methane was CH4 that is, one carbon atom forming bonds with 4 separate hydrogen atoms. This is where Kekulé enters the story.
Friedrich August Kekulé was born in 1829 in Darmstadt, Germany. He first studied architecture but became interested in chemistry and studied it with Justus von Liebig in Giessen and then in Paris. From 1854 to 1855 he was in London working as a laboratory assistant at St Bartholomew’s Hospital. In London he mixed with eminent chemists. He said that he developed his ideas about chemical structure at this time but denied that he had learned them from Frankland. Kekulé later described how he got his ideas while dozing on the top of a horse-drawn bus in Islington, London. He dreamt that he saw carbon atoms “gambolling” and joining together to form chains of two, three, four and more atoms. Then atoms of other elements, such as hydrogen, joined on to complete the molecules.
Kekulé left London for Heidelberg and then was appointed professor of chemistry at the University of Ghent in 1858. There he published the ideas that originated in his dream and began publication of a popular textbook. It was in this book that Kekulé introduced his “sausage” diagrams to show the structure of molecules with the number of bulges in each sausage-shaped atom equal to the valency of the element.
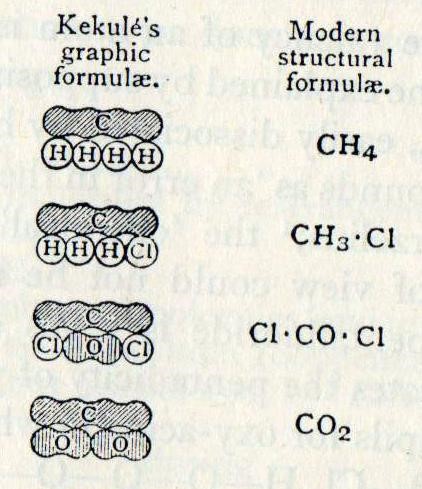
Fig.1 [A History of Chemistry vol.4, Partington, pub Macmillan, 1964 p.540 only use top and bottom, i.e. CH4 and CO2]
In a lecture at the Royal Institution in London in 1864 the well-known chemist August Hofmann entertained his audience using models of molecules. The models had wooden balls representing atoms joined by rods.
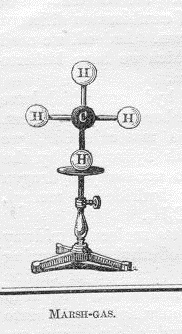
Fig. 2 Hofmann’s model of methane (CH4)
A year earlier the Edinburgh chemist, Alexander Crum-Brown had suggested that the valency of atoms could be shown as short lines sticking out of circles standing for atoms. When atoms were joined in molecules the lines could be linked up to form bonds. A year later Frankland and Crum-Brown suggested that double bonds could form between some atoms and Frankland simplified the diagrams by removing the circles and leaving just the symbols of the elements. Thus the structural formulae which are familiar today came into use.
The system worked well with most organic compounds where the carbon atoms were in chains. Benzene, however, caused a problem as with just 6 hydrogen atoms it was impossible to complete all the bonds formed by the carbon atoms unless there were a lot of very reactive double and triple bonds in the molecule. Benzene is not very reactive so it was reasoned it could not have triple bonds in its molecules
Kekulé persisted with his sausage diagrams and struggled with the benzene problem. Once again, according to his later memoirs, he fell into a doze in his Ghent study. This time he saw chains of carbon atoms wriggling like snakes until one bit its own tail and formed a loop. When he awoke he realised that he had solved the problem. The six carbon atoms of benzene were arranged in a hexagonal ring with a hydrogen atom attached to each carbon. First he drew the structure using his sausages but then made other diagrams showing more clearly the hexagonal arrangement.
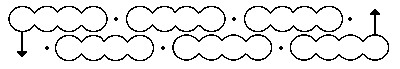
Fig.3. Kekulé’s sausage diagram for the ring structure of benzene
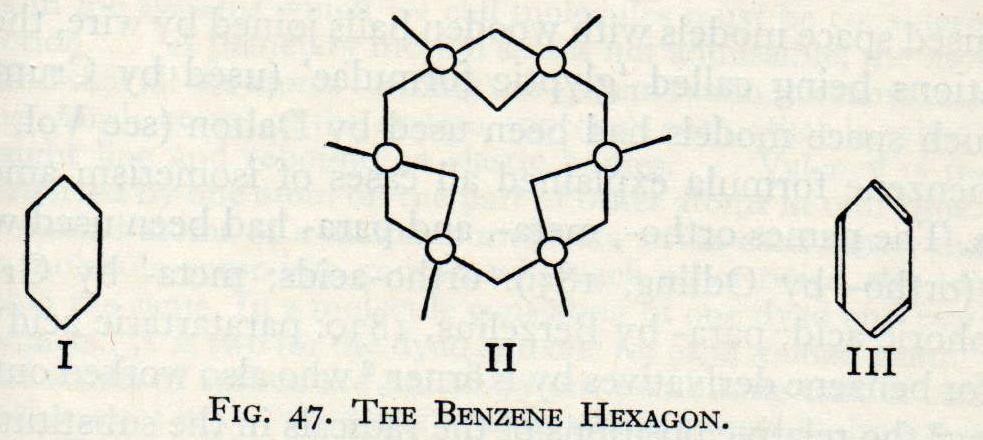
Fig.4 Other diagrams of benzene used by Kekulé
The structure contains three double bonds which should make benzene pretty reactive. Kekulé got over this by saying that the structure flips between the two possible arrangements of the double bonds so that no bonds are ever fully double.
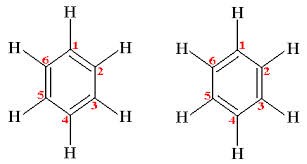
Fig.5 The two alternative forms of the benzene structure as suggested by Kekulé
Kekulé described his idea in the Paris paper of 1865 and the ring structure of benzene was quickly accepted. Benzene is the simplest of a whole class of “aromatic” carbon compounds so the ring structure appears in many substances. Kekulé moved to the more prestigious university in Bonn in 1867 where he remained until his death in 1896 having enjoyed a great deal of fame for his discovery.
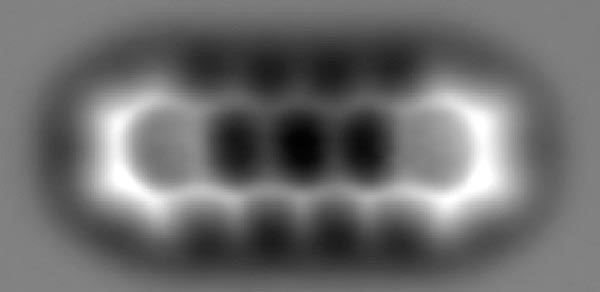
It wasn’t until 1929 that the hexagonal structure of benzene was proved. In one of the first uses of X ray crystallography to determine a molecular structure, Kathleen Lonsdale at the Royal Institution proved that the six carbon atoms in the benzene ring were arranged in a planar (flat), symmetrical hexagon. Lonsdale was the first woman to become a Fellow of the Royal Society. In 2009 atomic force microscopy was used to make the atoms in benzene rings visible for the first time.
Activities
- Like many chemists in the nineteenth century as well as today, August Kekulé studied and worked in a number of different places before settling in Ghent and Bonn. Why is this important?
- Find out more about the lives and work of the people mentioned in this article – August Kekulé, Michael Faraday, Edward Frankland, Alexander Crum-Brown, Kathleen Lonsdale
- Why were the models and diagrams inspired by Crum-Brown so useful to chemists that they are still used today?
- Kekulé didn’t reveal his stories about making his discoveries in dreams until years afterwards. Why do you think this was? Do you believe Kekulé’s stories?
- Why was it important for chemists to understand the structure of benzene?
Bibliography
- Oxford Dictionary of Scientists, OUP
- A History of Chemistry, J.R. Partington, Macmillan, 1964
- Faraday’s sample of benzene at the RI http://www.rigb.org/our-history/iconic-objects/iconic-objects-list/faraday-benzene
- Article written on the centenary of the discovery of benzene http://pubs.acs.org/doi/abs/10.1021/ed003p1248
- Kathleen Lonsdale http://www.rsc.org/diversity/175-faces/all-faces/dame-kathleen-lonsdale-dbe-frs
Pictures
Fig.1 [A History of Chemistry vol.4, Partington, pub Macmillan, 1964 p.540 only use top and bottom, i.e. CH4 and CO2]
Fig.2 Hofmann’s model of methane (CH4)
https://webspace.yale.edu/chem125/125/history99/6Stereochemistry/models/H6c2h4.JPG
Fig.3 Kekulé’s sausage diagram for the ring structure of benzene
http://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2014/05/kekule-sausage.gif
Fig.4 Other diagrams of benzene used by Kekulé
[A History of Chemistry vol.4, Partington, pub Macmillan, 1964 p.555]
Fig.5 The two alternative forms of the benzene structure as suggested by Kekulé
http://canov.jergym.cz/mechanic/pravidl2/lad/r_soubory/benzen2.gif
Fig.6 Atomic force microscope picture showing five benzene rings joined together
http://images.iop.org/objects/phw/news/13/8/19/afm1.jpg