Michael Faraday, one of the UK’s greatest scientists, died in 1867 at the age of 76. That was at the peak of the Steam Age. Railways linked cities, towns and many villages; steam engines powered factories and steamships crossed the oceans. The Electric Age that Faraday helped to launch was just beginning. The electric telegraph carried messages across countries. The first transatlantic cable had been laid so the telegraph connected continents too. Electric arc lights were beginning to replace paraffin lamps in lighthouses (Faraday had a lot to do with that) and the invention of the electric light bulb was not far off. Already electric motors had powered experimental boats and vehicles and electric generators were being developed to provide the power the world needed.
Faraday wasn’t aware of many developments in the outside world during his last three or four years. Since the eighteen forties he had suffered from mental problems. His short-term memory became worse and worse so that he was unable to remember even the contents of a letter from a scientific colleague once he’d finished reading it. The cause of his problems could have been one of the forms of dementia we are familiar with today although some people think it may have been due to mercury poisoning. Mercury featured a lot in Faraday’s work as it was used in instruments as well as in chemical experiments.
His most important work was done between 1820 and 1840 on electromagnetic induction and electrolysis. This earned him the respect of scientists all over the world and of the British government and Queen Victoria. While his discoveries made some people rich, Faraday just took a salary from the Royal Institution of London and he refused all honours. He remained simple Mr Faraday until his death in a house at Hampton Court granted to him by Queen Victoria when he was unable to afford to buy a house himself. He even refused to have his body buried in Westminster Abbey alongside Isaac Newton, preferring a private funeral performed by members of the small Christian sect called the Sandemanians that he had belonged to all his life.
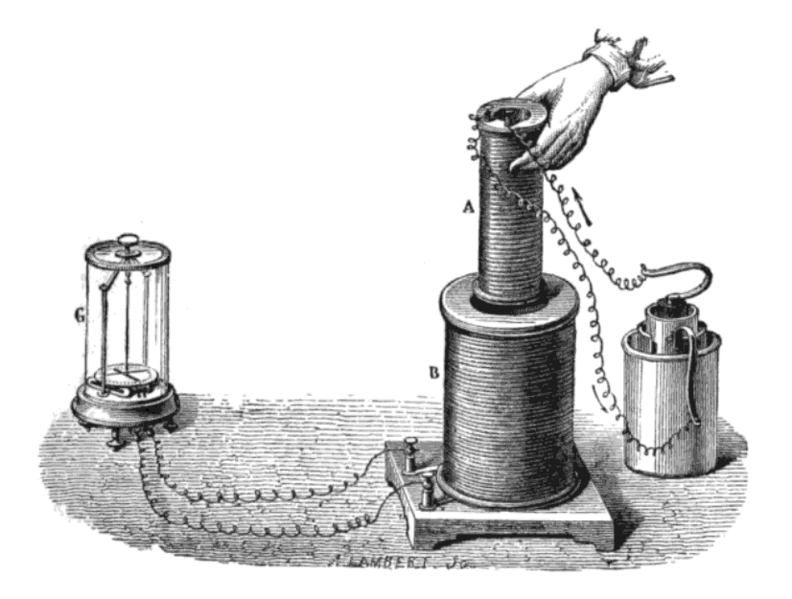
Faraday’s most significant discoveries were in physics. In 1831 he showed that an electric current in one coil of wire could induce a current in another coil. The next year another experiment showed that an electric current could be generated by moving a magnet in and out of a coil of wire. These discoveries lead to the transformer and electricity generator which are the foundation of the electricity generating industry.
Faraday was a chemist before he was a physicist. His first employer at the Royal Institution was Humphry Davy who in the 1800s used the nearly invented electric battery to extract the metals, sodium, potassium, magnesium and calcium from their salts. In 1834 Faraday returned to the study of electrolysis. He discovered the Laws of Electrolysis which determine how the amount of substance deposited depends on the current and the charge on the ions. With William Whewell he developed a new vocabulary to describe electrolysis including the terms, cation, anion, cathode and anode.
In the 1820s Faraday carried out other chemical experiments. One resulted in the discovery of the hydrocarbon, benzene, which Faraday called bicarburet of hydrogen. Faraday joined the Royal Institution as Davy’s assistant in 1813. Shortly after, he spent almost eighteen months accompanying Davy and his wife on a tour of Europe. They visited scientists in many cities even though the war against Napoleon was still being fought.
Faraday’s interest in science had been fired while apprenticed as bookbinder. He attended lectures including some by Davy at the Royal Institution and wrote up everything he heard. He sent a copy of his notes to Davy who realised that he would be a useful assistant, and later, successor.
Faraday spent almost fifty years at the Royal Institution. Apart from research, his duties included delivering lectures to the public. He gave his first lecture to children in 1826. The Christmas Lectures for children became very popular and from the 1840s it was an annual event for Faraday. Giving up the lectures in 1861 was one of the saddest events for him as he contemplated the end of his working scientific life. One of his most popular lectures, much repeated, was The Chemical History of a Candle, which shows how important he considered chemistry.
Faraday was no mathematician and his theoretical ideas on electricity and magnetism were visual – he drew diagrams of lines of force. Nevertheless, his experimental work and his ideas inspired many physicists and inventors that followed him. His work is still recalled at the Royal Institution in London and we should think of him when we use an electrical device whether it runs on mains electricity or batteries.
Activities
- Look at the timeline of Faraday’s life (see below) and note the discoveries that he made.
- Imagine life without electrical power available at the touch of a switch. Discuss the importance of Faraday’s discoveries in electromagnetic induction.
- Faraday didn’t patent the transformer or electric generator. What effects did this have?
- Faraday was respected by scientists across the world and he became a member of many foreign scientific societies but he refused all honours at home. Why do you think this was and what effect do you think it has had on our memory of Michael Faraday?
- Faraday had only simple schooling and never went to university. How was he able to achieve so much success in science?
- Faraday considered his lectures to children and young people very important. Do you agree? What makes a lecture interesting to children?
- Faraday’s final research was on trying to unify electromagnetism and gravity, which also filled Einstein’s last years and occupies many scientists today. His last scientific paper was refused publication because all his experiments failed to find a connection. Do you agree that experiments that don’t work are a waste of time?
- Two units are named after Faraday – the Farad and The Faraday Constant. Find out what they are.
- Find out what Faraday’s Laws of Electrolysis tell us.
- How do you think we should celebrate Michael Faraday’s life?
Bibliography
Michael Faraday, by L. Pearce Williams, pub. Da Capo Press
http://www.rigb.org/our-history/michael-faraday/about Royal Institution website including a Faraday timeline.
Photo Sources
Faraday’s discovery of electromagnetic induction. https://commons.wikimedia.org/wiki/File:Induction_experiment.png
Michael Faraday. https://commons.wikimedia.org/wiki/File:Faraday-Millikan-Gale-1913.jpg
By Peter Ellis