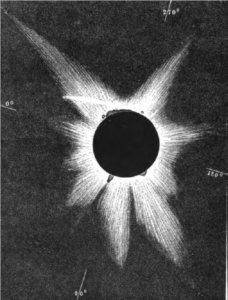
In August 1868 a total solar eclipse was predicted to cross India and Thailand. Astronomers from around the world travelled by land and sea to observe the Moon covering the Sun. Many were keen to use a new instrument, the spectroscope, to examine the Sun’s corona.
Isaac Newton used a prism to split light from the Sun into the many-coloured spectrum. In 1814, the German glass and lens maker, Fraunhofer, discovered that the spectrum of the Sun had thin dark lines across it. It was 1859 before an explanation for the lines was given by Gustav Kirchof. He said that they were due to absorption of certain wavelengths of light by elements present in the Sun. In 1860, Kirchof’s colleague at Heidelberg University in Germany, Robert Bunsen, was examining the light given out when compounds were heated. Bunsen’s assistant had devised a special burner (ever since known as the Bunsen burner) to give a flame that was colourless itself. Kirchof suggested passing the light from the compounds through his spectroscope. They were thus able to record the pattern of coloured lines in each element’s spectrum and measure the wavelengths of light emitted. They soon found that the Fraunhofer lines matched the spectrum of known elements, such as hydrogen and sodium, showing that these elements were present in the Sun. They also discovered two new elements, caesium and rubidium, in mineral water by their distinctive spectra. Soon, other chemists were using the spectroscopic method to look for new elements on Earth while astronomers pointed similar instruments at the Sun and stars.
Norman Lockyer was 32 years old in 1868 and working as a civil servant in London. He was already a respected amateur astronomer with a telescope in his garden in Wimbledon. He was particularly interested in sunspots and prominences (huge flares of bright gas that burst from the surface of the Sun). He wasn’t part of the team sent out by the Royal Society and Royal Astronomical Society to observe the Indian eclipse, but he waited eagerly for news of the results. Captain John Herschel (grandson of William Herschel, the discoverer of the planet Uranus and infra-red radiation) lead one of the teams. He reported using a spectroscope to observe the corona of the Sun during the eclipse. Spectral lines were observed but the measurements Lockyer received by telegraph were not very accurate or useful.
Also present at the eclipse was French physicist, Pierre Janssen, a keen traveller who had already visited Peru. He collected more accurate measurements of the spectra of the corona. Some lines he matched to the element hydrogen, but others Janssen was unable to match with known elements. He sent his results to Lockyer amongst others. Lockyer had just taken delivery of a new spectroscope to attach to his telescope. This enabled him to focus on the prominences of the Sun without waiting for an eclipse. He was quickly able to repeat Janssen’s measurements and confirm that most of the gas of the prominence was hydrogen, but he also observed the lines of the unknown element. Lockyer made many more observations of the Sun’s spectrum and corresponded with the chemist Edward Frankland who did experiments on the spectra of various elements at very high temperatures and pressures. Lockyer concluded that there must be an element that only existed in the Sun and he gave it the name Helium from the Greek word for the Sun, helios. The “ium” ending was because Lockyer assumed it must be a metal like most other elements. Janssen and Lockyer were both credited with the discovery of the new element.
Not everyone agreed that helium existed. After he published his Periodic Table in 1869, Mendeleev wouldn’t accept that another metallic element fitted between hydrogen and lithium. The existence of helium remained in doubt as none was found on Earth and the spectrum of the Sun was the only evidence. Lockyer went on to be the founding editor of the science magazine, Nature, and in 1885 became the first professor of astronomical physics at the Royal College of Science, now part of Imperial College, London. Janssen continued to travel the world observing eclipses. In 1875 he was appointed director of a new astrophysics observatory just outside Paris. Janssen and Lockyer remained close friends.
It wasn’t until 1895 that helium was found on Earth. William Ramsay, professor of chemistry at University College, London received a sample of a uranium mineral called cleveite (named after its discoverer, Per Cleve). He had heard that it gave off an unusual gas when heated. Ramsay collected the gas and his colleague, William Crookes, examined its spectrum. It matched the lines Lockyer and Janssen had observed in the Sun. The previous year Ramsay had discovered argon in the air. Now with his assistant Morris Travers they distilled a sample of liquid argon obtained by removing oxygen and nitrogen from air. A few gases were given off. When cooled with liquid hydrogen they obtained a small amount of helium. They also discovered the previously unknown gases, neon, krypton and xenon.
Ramsay had shown that helium existed on Earth but was rare and instead of being a metal it was an unreactive gas. He suggested to Lockyer that the name be changed to helion, but Lockyer did not reply. The element remained helium. Ramsay’s discoveries did, however, answer Mendeleev’s objections. Helium and the other noble gases formed a new group in the Periodic Table.
In 1903, a company drilling for oil in Kansas, USA, struck a well of natural gas. Unusually, the gas didn’t burn. In fact, it only contained 15% methane. Most of the rest was nitrogen but 12% proved to be helium. Helium was found in other sources of natural gas and so was not rare after all. Also in 1903, Ramsay, working with the radioactivity expert Fred Soddy, found that radium, discovered five years earlier by Marie Curie, gave off helium. Four years later, Ernest Rutherford proved that the helium was formed from the alpha particles emitted by radium and other radioactive elements. In fact, most of the helium found in the Earth is the result of radioactive decay, because while helium was formed, with hydrogen, shortly after the Big Bang, any that was on the Earth when the planet was formed will have escaped into space. That is the fate of all the helium in party balloons that are released in great numbers.
Helium atoms are just twice as heavy as hydrogen molecules so helium-filled balloons have almost as much lift as balloons inflated by hydrogen. As helium does not burn it is also safer. When airships were developed by Germany, hydrogen was used as there was little helium in Europe. In the USA all the helium collected from oil and gas wells could be used. In 1925, the USA government set up the National Helium Reserve to stockpile helium for future airships. However, following disasters like the crash of the airship Hindenburg (filled with hydrogen), and improvements in aircraft the enthusiasm for airships died. The USA stocks of helium continued to increase even when liquid helium found a use as a coolant for liquid hydrogen and oxygen rocket fuels. The US government decided to sell off the stocks in 1996, which was when the gas became readily available for party balloons. This coincided with the growth in the use of liquid helium to cool the superconducting magnets in everything from MRI scanners to the Large Hadron Collider at CERN. There are other uses of the gas too. With oil wells running out, a shortage of helium developed. However, a huge reserve of helium has recently been discovered in Tanzania so the supply is secure, for now. The rate of replacement of helium by radioactivity is less than its consumption, particularly if we continue to release balloons filled with it into the atmosphere. Frivolous use of this vital, if unreactive element, discovered 150 years ago, should be reduced.
Activities
- Why wasn’t helium discovered before 1868?
- Why was the discovery of helium not accepted by some scientists?
- Why was Ramsay’s work important in proving helium to be an element?
- Do you think the name of the element should be helium or helion?
- Why is practically none of the helium that was present when the Earth was formed still in the atmosphere now?
- How do you think the history of aviation might be different if Europe, and Germany in particular, had access to cheap supplies of helium in the 1920s and 30s?
- How are alpha particles and helium related?
- “Helium is too valuable an element to be wasted in party balloons.” Discuss this statement.
- Find out more about the life and work of Norman Lockyer, Pierre Janssen and William Ramsay.
Bibliography
150 years of helium, James Mitchell Crow, Chemistry World pub. Royal Society of Chemistry p.50 vol. 15 no. 8 Aug 2018
Contributions to Solar Physics, Norman Lockyer, pub. Macmillan, 1874 (available on https://books.google.com)
Nature’s Building Blocks: An A-Z Guide to the Elements, John Emsley, pub. Oxford 2011
The History of Chemistry, John Hudson pub. Macmillan 1992
Oxford Dictionary of Scientists, pub. Oxford 1999
The Lost Elements: The Periodic Table’s Shadow Side, Fontani, Costa & Orna pub. Oxford 2015
A History of Chemistry, vol.IV, J.R. Partington 1964
Wikipedia
Illustrations
Illustrations taken from Contributions to Solar Physics, Norman Lockyer, pub. Macmillan, 1874 (available on https://books.google.com)
- Drawing of the 1868 solar eclipse showing the corona and prominences.
2. A spectroscope similar to the type Lockyer attached to his telescope to observe the spectrum of the Sun, with five prisms for separating the wavelengths of light.
3. An astronomer peers through a spectroscope attached to a refracting telescope, such as Lockyer and Janssen would have done.
By Peter Ellis