Students of chemistry will be familiar with dot and cross diagrams showing pairs of electrons in simple molecules. Gilbert Lewis proposed these diagrams and the idea that bonds between atoms were formed by pairs of shared electrons in a paper called “The Atom and the Molecule” in 1916.
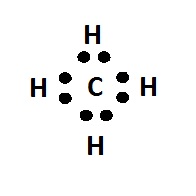
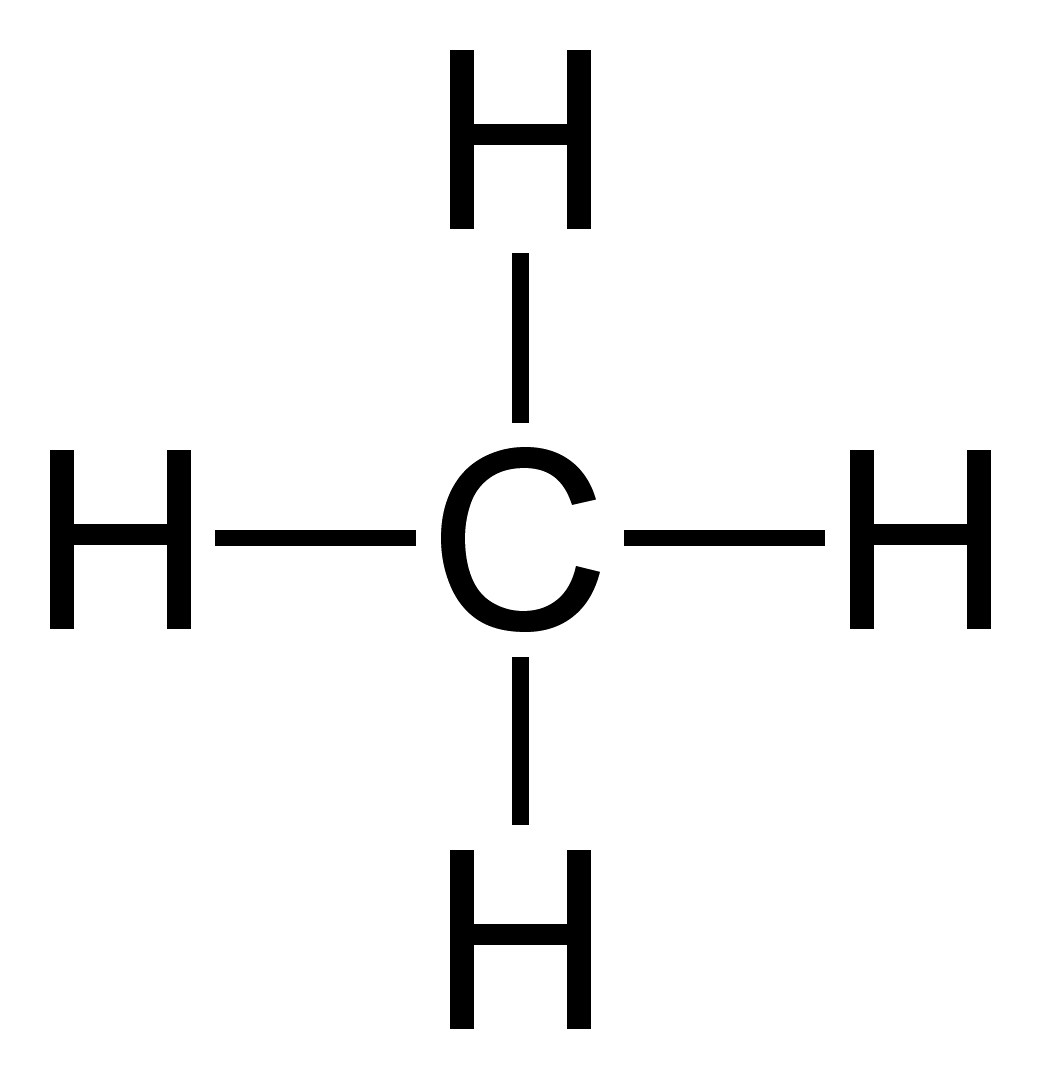
[fig.1 dot diagram of methane molecule]
Science in 1916
In 1916 the world was at war. The Battle of the Somme in France was one of the biggest battles in the trench warfare and hundreds of thousands died. Many scientists had left their laboratories to fight on the frontline, such as Henry Mosely who was killed in 1915. Others put aside their research projects to assist the war effort. For example, Ernest Rutherford worked on sonar for detecting submarines underwater and Marie Curie trained men and women to operate mobile X ray machines for photographing wounded soldiers.
The USA was officially neutral but sent supplies to Britain and its allies. It joined the war in 1917.
Gilbert Lewis and Irving Langmuir
Gilbert Lewis was born in 1875 in Massachusetts, USA and studied chemistry at the state’s prestigious Harvard University. Following a short period of study in Germany he returned to teach at Harvard in 1902.

During 1905 he worked for a short time in the Philippines where he developed a liking for the large cigars they made there. Then he took a research post at Massachusetts Institute of Technology. In 1912 he was invited to take over the Chemistry Department at the University of California at Berkeley where he remained for the rest of his life.
Lewis was a rather shy man but could be rude to people he didn’t get on with. He didn’t like teaching so avoided it while running his department, but he made sure that those who did teach helped their students to understand the subject instead of just learning facts by heart. He didn’t like mixing with groups of people not well-known to him but relished the weekly seminar in his department where everybody had a say on each other’s progress. He disapproved of colleagues who mixed university work with advising industry and removed courses in applied science from his university’s curriculum. He was a rather boring lecturer but considered his published papers sufficient to spread his ideas. Lewis chain-smoked the large cigars he imported from the Philippines in large quantities.
Lewis’ main scientific interest from 1898 was in chemical thermodynamics, the study of energy changes in chemical reactions. His achievements were recognised by many other scientists. In 1923 he wrote a book on the subject that was used by many students.
Irving Langmuir was born in 1881 in New York. He first studied metallurgy and then went to Germany and took his doctorate in chemistry in 1906. In 1909 he started work with the General Electric Company where he stayed all his working life. His research resulted in many technological improvements to light bulbs and the valves that were increasingly in demand for radios and amplifiers. The company made big profits from his work. He also studied surface films formed when oil spreads out on the surface of water, and in reactions on the surface of a solid catalyst. Langmuir was a good speaker and popular with other scientists.
The Atom and the Molecule
Following J J Thomson’s discovery of the electron in 1898, many scientists wondered how electrons were arranged in the atom. It was realised that electrons could be transferred from one atom to another to form ions. The arrangement of ions in a solid compound such as sodium chloride was observed using X-rays in the nineteen-teens. Scientists also wondered how the number of electrons in an atom was related to the place of the element in the Periodic Table.
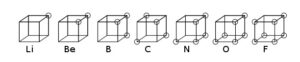
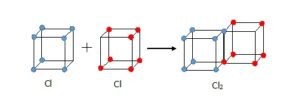
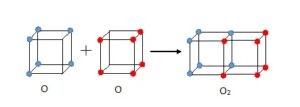
In 1902, Gilbert Lewis had the idea that electrons might be arranged at the corners of a cube. Moving across a row (or period) of eight elements in the Periodic Table the corners would be filled up. His boss at Harvard thought the idea was “twaddle” but Lewis persisted with it. The 1913 Rutherford-Bohr model of the atom had electrons moving in fixed orbits but it was difficult to see how this could explain molecules where atoms were held together by some strange bond. An example was a chlorine molecule where two atoms of chlorine each with seven electrons in their outer shell were joined together. In 1915. Lewis realised that his cube models suggested a way that atoms could link. He overlapped two chlorine cubes along an edge so that the electrons at those corners where shared between two cubes. Similarly, in an oxygen molecule, the cubes of two oxygen atoms could be joined at a face with four electrons shared. In both molecules all the atoms now had at least a share of eight outer electrons.
[fig.2 diagram of Lewis cubes for selected atoms, chlorine molecule and oxygen molecule]
For larger molecules the overlap of the cubes became difficult to draw so Lewis introduced his simpler dot diagrams. The dot represented an electron and a shared pair of electrons between two atoms was the bond. One objection to the idea was that being negatively charged, a pair of electrons should repel rather than share a space in a molecule. Lewis was confident that it would be discovered that at short distances the repulsion would not happen.
[fig.3 dot diagrams of chlorine and oxygen]
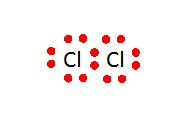
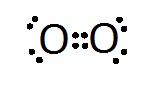
In 1916, Lewis published his ideas in a paper called “The Atom and The Molecule”. He emphasised the importance of pairing electrons in the outer shell of atoms when forming compounds.
Dispute
Lewis’ paper was ignored by most scientists as most, particularly organic chemists, thought it solved a problem they didn’t know they had. He was not one for publicising his work in packed lecture rooms but he did discuss his ideas with Langmuir when they met at a meeting in New York. Soon the talk was of war and in December 1917 at the age of forty-two, Lewis enlisted in the United States Army. He was sent to France to observe the use of poison gases. Lewis was put in charge of training soldiers how to survive attacks. He reduced the number of allied soldiers killed and injured in gas attacks but he also suggested more effective ways of using poison gas against enemy soldiers.
Meanwhile Langmuir had taken to Lewis’ ideas and used his cube models in his own work. At first he missed the point about the pairing of electrons in bonds but “rediscovered” it in 1919. He suggested his “octet theory” i.e. that ions and atoms in molecules have eight electrons in their outer shell just like noble gases. Langmuir publicised his work in journals and popular lectures and while he referred to Lewis, the theory quickly became known as the Lewis-Langmuir theory. Lewis was angry at Langmuir’s apparent “theft” of his ideas. Letters were written and other scientists drawn into the dispute. When they looked back at Lewis’ 1916 paper it was realised that the all the important ideas were there and the theory of what became called covalent bonds was Lewis’ alone. Langmuir acknowledged that Lewis had priority but Lewis maintained a grudge.
The dot diagrams became very popular as they helped chemists at all levels show how the electrons in molecules were arranged and the bonds that could be formed between atoms. Crosses as well as dots are now often used to indicate the atoms to which the electrons belong to, although, of course, electrons cannot be distinguished. In 1923 Lewis wrote a short book, “Valence and the Structure of Atoms and Molecules” which added detail to his ideas.
The missing Nobel Prize
After 1923, Lewis left his work on thermodynamics and the chemical bond and took up a new study. He thought about the properties of light, a topic that was of interest to theoretical physicists. He gave the name photon to a particle of light. In the 1930s Lewis changed course again, joining the growing subject of nuclear chemistry and was involved in the race to make heavy water (water molecules containing atoms of the deuterium isotope of hydrogen).
On a number of occasions, he was nominated for a Nobel prize for his earlier work on thermodynamics and later for his theories on chemical bonds but he was never awarded the prize. The reasons were partly that having left one area of science to do research in another he didn’t keep up to date. The other reason was a result of his personality. He had annoyed many scientists by dismissing their ideas as old-fashioned or simply wrong. Some of these scientists had influence over the award of the Nobel prizes so they blocked or delayed his nominations. Meanwhile many of the chemists he trained at Berkeley went on to earn honours and Nobel prizes. In 1932, his rival Langmuir received the Nobel Prize for Chemistry.
Lewis continued to run the department at Berkeley but became increasingly isolated. At the age of 65 he had to retire from his leadership roles but he continued to do research. During the 2nd World War, he was disappointed not to be called on to join the Manhattan Project that built the atom bomb. One day in 1946, Langmuir visited Berkeley to receive an award from the university. Lewis was invited to lunch with his old rival. Afterwards, he was found dead in his laboratory that was filled with hydrogen cyanide gas. It was never decided whether he had committed suicide because of depression and disappointment or whether the flask pf cyanide had been knocked over when he had a heart attack caused by poor diet, lack of exercise and smoking innumerable cigars.
The Chemical Bond

One of Lewis’ research workers at Berkeley was Linus Pauling. Pauling built on Lewis’ ideas and became a leading expert on chemical bonds. He was awarded a Nobel Prize for his work and he wrote a book called “The Nature of the Chemical Bond” dedicated to Gilbert Lewis which was used by chemistry students for many years. It makes a lot of use of Lewis’ dot diagrams.
Tasks
1 Find out more about the lives and work of Gilbert Lewis, Irving Langmuir and Linus Pauling.
2 Draw Lewis dot diagrams for
a. The ions in magnesium oxide (MgO)
b. Molecules of ammonia (NH3), carbon dioxide (CO2) and phosphorus trichloride (PCl3)
3 What differences were there between Lewis’ cubic model of the atom and Rutherford-Bohr nuclear atom?
4 Why did Lewis’ ideas about bonds became known as the Lewis-Langmuir theory? Why was Lewis angry about this name? What do you think he might have said to Langmuir?
5 Lewis was interested in many different topics in chemistry and physics. What reasons are there for changing subjects being a good or bad thing for scientists to do?
6 Gilbert Lewis is not referred to much in textbooks nor did he win a Nobel Prize. Do you think he deserves more recognition?
Bibliography.
Cathedrals of Science, the personalities and rivalries that made modern chemistry, by Patrick Coffey, pub. Oxford University Press 2008.
Unpublished talk by Patrick Coffey at a meeting of the Historical Group of the Royal Society of Chemistry, 23rd March 2016
Oxford Dictionary of Scientists, pub OUP
The Nature of the Chemical Bond, by Linus Pauling pub. Cornell University Press (first published 1939, third edition published 1960 ninth printing 1980)
Photo sources:
Irving Langmuir – https://commons.wikimedia.org/wiki/File:Langmuir-sitting.jpg
Linus Pauling – https://commons.wikimedia.org/wiki/File:L_Pauling.jpg
Peter Ellis