I had intended, as mentioned in the previous posting, to cover Mass Extinction Events in this next blog. However, a newspaper story caught my eye. The story concerns a theme which I have returned to in several of the previous posts; it’s that of regulation of protein synthesis and of course the crucial role that this plays in cancer. The research reported in the newspaper article seems so important and also biologically so interesting that the intended blog goes on the back burner and in this posting we’ll have a look at the research behind the news story instead.
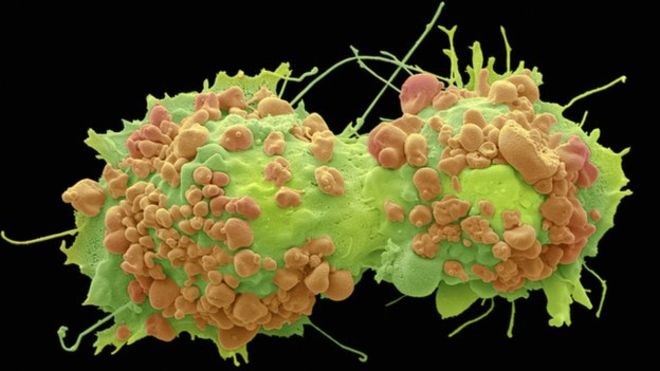
It’s been known for a long while that cancer cells behave differently than their normal counterparts. In the body (in vivo), they push aside normal cells, invade healthy tissue and take over blood supply. In vitro, i.e. in cell culture in the laboratory, their behaviour is similar. Normal simple squamous epithelial cells such as those lining the air sacs of the lungs are one cell thick both in living vertebrates and when they are in vitro. Cell cultures of these normal cells can live and divide in glassware, but cease cell division once the space has been used up, for example the Petri dish or glass bottle surface has been covered by actively dividing cells. It is also possible to grow cancer cells in glassware; in fact cancer cells often are long lasting and have been used as “cell lines” which have been studied extensively over several decades. An example of this is the cell line HeLa. These cells originated in a cervical tumour in Henrietta Lacks who died from the cancer on 4th October 1951. Henrietta died 64 years ago, but cells from her tumour are still alive today and appear to be immortal. Such cancer cells do not stop dividing and, unlike healthy tissue, they do not form ordered layers, instead they form heaps of disordered cells. Cancer research has, amongst many other directions, studied the relationship of cells one to another. This is because of the marked difference between normal and cancer cells in their interaction with neighbouring cells and with their environment. Adhering to a structure such as a Basement Membrane or to each other is crucial to the three dimensional design of the tissue; just as in architecture where similarly sized living spaces adhering to each other in organised layers will generate a block of flats or adherence along one side results in a row of semis. The new research findings are a result of this focus.
The research team at Mayo Clinic in Jacksonville, USA have been investigating the role of two adhesion proteins, E-cadherin and p120 catenin which are engaged in anchoring cells to each other and to structures of their environment. For some time these molecules were considered to be effective tumour suppressors, however reports also showed high levels of the adhesion proteins in cells of cancerous tumours and more worryingly, there was evidence that E-cadherin and p120 catenin promoted tumour growth.
The Jekyll and Hyde properties of E-cadherin and p120 catenin was found to be flipped by another protein, PLEKA7. The recent research by Professor Anastasiadis’ team at the Mayo Clinic found that in the presence of PLEKA7 the adhesion proteins E-cadherin and p120 catenin suppress cancer growth, but when this PLEKA7 is lost, these adhesion proteins switch role and become tumour promoting.
The relation between the adhesion proteins and unrestricted growth of cancer cells is thought to be related to a group of polynucleotides known as micro RNAs, (miRNAs) which were first discovered in 1993. It is thought that in the absence of PLEKA7, adhesion proteins E-cadherin and p120 catenin in some way destabilise, the normal activity of miRNAs. To see how this may affect cancer growth we first need to consider the normal role of miRNAs.
miRNAs – Structure
These are, as their name suggest, small lengths of RNA folded to form a structure reminiscent of Transfer RNA. Their shape has been likened to a hair-pin, they look more like a hair grip to me, but hair control is not my special subject, so here is a diagram:
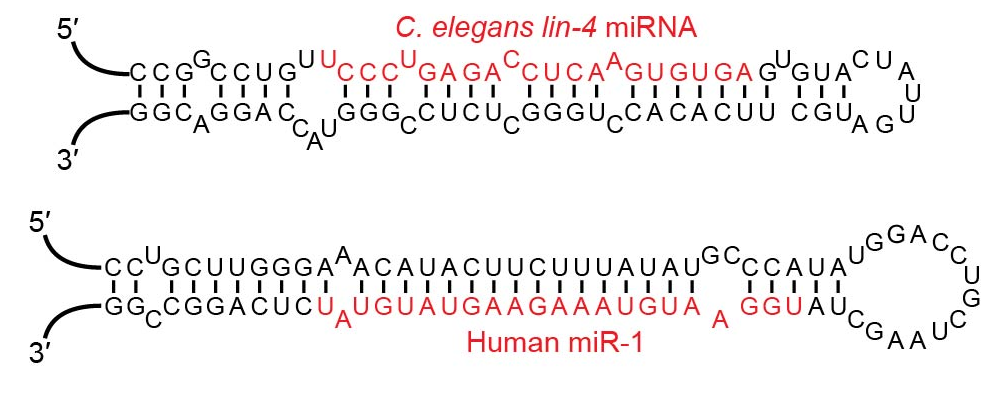
Examples of microRNA stem-loops showing their “hair-pin” like structure. Notice the similarity of these two examples even though one is from a nematode and the other human. This is indicative of a very ancient origin.
Just so you can see the difference in structure and therefore evolutionary history between Homo sapiens and C. eligans (shortened name for Caenorhabditis eligans) a nematode commonly found in the soil of temperate regions. Very elegant too it appears to be, particularly if you have a fond spot for nematodes. For a view of the other species, try a mirror!
miRNAs – Function
miRNA are non-coding molecules, that is they are not involved in the synthesis of proteins as is the case of the more familiar messenger RNA (mRNA), instead they are involved regulation of gene expression post-transcription. What this boils down to is the miRNAs interfere with already transcribed mRNA as shown in this next diagram:
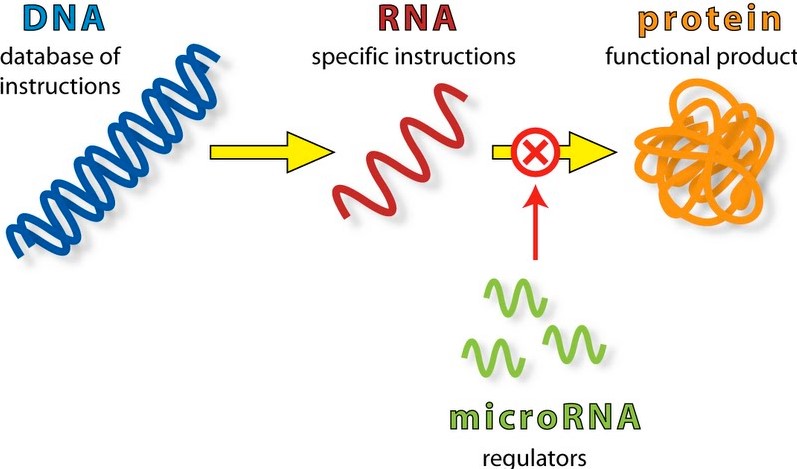
The top line of the diagram shows the normal pathway for protein synthesis; the green part shows the stage in the pathway where miRNAs interfere with the transcribed mRNA, preventing (or moderating) the translation of mRNA into protein. The exact method by which this happens is not known, but naturally it is the focus of a deal of research. See the following URL for further information: https://en.wikipedia.org/wiki/MicroRNA and also a more detailed diagram: The Formation and Function of Micro RNAs (Image 5: source below).
Don’t worry too much over the detail, notice the following stages:
- The hairpin shape of the primary miRNA shown top left
- The action of the dicer enzyme which splits off the loop of the miRNA, which then leaves one of the strands to join to an Argonaute protein. The remaining strand is discarded. The Argonaute protein offers the miRNA fragment to the messenger RNA.
- and 4. Plant cells and animal cell differ in their handling of the single stand of miRNA, in both the miRNA combines with mRNA but base pairing mismatches occur between nucleotides in the case of animal cells whereas mismatches do not happen in plant cells. The net result in both cases is the destruction of the mRNA strand and therefore termination of its ability to translate the production of protein at the Ribosome
The functionality of miRNAs as tumour suppressors is seen to be through their disruption of protein synthesis as mentioned in the mechanism above. This seems plausible because if cells are rapidly proliferating, protein production either for enzymes or for structural components such as in cell membranes, must be crucial and probably the controlling factor for the rate of tumour growth.
Well that’s all for now; this research field seems likely to yield more fascinating results fairly soon, so it seems possible that it may again be the subject of a post. Before we go we should recognise that Professor Anastasiadis has another use for this research, as he mentions in the YouTube video referenced below. One of the major difficulties experienced in surgery is identifying cancerous material during operations. His research offers a method to quickly distinguish between cancerous and normal tissue, possibly to a cell by cell resolution.
Toodle-oo,
Sphenodon
Reading around the subject
As you can imagine, there’s been a lot of interest shown by the media, here’s a selection. If nothing else, you should look at the YouTube video by Professor Anastasiadis.
These pages are useful general background:
- http://www.telegraph.co.uk/news/science/science-news/11821334/Cancer-cells-programmed-back-to-normal-by-US-scientists.html
- http://www.bbc.co.uk/news/health-34041374
- http://www.medicalnewstoday.com/articles/298513.php
- http://elitedaily.com/news/scientist-turn-human-cancer-cells-back-normal/1193059/
- http://scienceblog.cancerresearchuk.org/2015/06/18/turning-off-bowel-cancer-as-easy-as-apc/
- http://news.nationalpost.com/news/world/u-s-scientists-successfully-turn-human-cancer-cells-back-to-normal-in-process-theyre-hopeful-can-one-day-switch-off-disease
- http://www.kurzweilai.net/how-to-reprogram-cancer-cells-back-to-normal
- http://www.geek.com/science/possible-kill-switch-for-cancer-cells-discovered-by-scientists-1632211/
Read the original paper by Professor Anastasiadis’ team here.
Background to early research on miRNAs:
Some questions that you might like
- The diagrams shown here of miRNA from cells of C. eligans and Homo sapiens are remarkably similar. Why should this be surprising and what does the similarity indicate about their historical origin? Thinking of the origin of life, what does the common use of nucleotide sequences as a genetic code indicate? There are several answers to this question, so initially working as a team might be a useful ploy to make sure you have a many sided approach in your final answers.
- Draw a diagram to help a non-specialist understand the role of miRNAs in the control of cell proliferation.
Images sources:
Image 1: credit: Steve G Schmeissner/Science Photo Library
Image 2: by VTD – Own work. Licensed under CC BY-SA 4.0 via Commons – https://commons.wikimedia.org/wiki/File:Examples_of_microRNA_stem-loops.jpg#/media/File:Examples_of_microRNA_stem-loops.jpg
Image 3: credit: “Adult Caenorhabditis elegans” by The original uploader was {{{realname}}} at English Wikipedia(Original text: Zeynep F. Altun, Editor of www.wormatlas.org) – Transferred from en.wikipedia to Commons.(Original text: Donated by Zeynep F. Altun). Licensed under CC BY-SA 2.5 via Commons – https://commons.wikimedia.org/wiki/File:Adult_Caenorhabditis_elegans.jpg#/media/File:Adult_Caenorhabditis_elegans.jpg
Image 4: credit: Firefly BioWorks
Image 5: “MiRNA” by Kelvinsong – Own work. Licensed under CC BY 3.0 via Commons – https://commons.wikimedia.org/wiki/File:MiRNA.svg#/media/File:MiRNA.svg