by Peter Ellis

The 2019 Nobel Prize for Chemistry has been awarded for research that all of us use – the lithium battery. Mobile phones, laptops, cameras, hearing aids, even electric toothbrushes, rely on rechargeable lithium-ion batteries. Electric vehicles such as the Tesla and Nissan Leaf also could not run without them. Three men have been picked out as responsible for the discoveries that made lithium-ion batteries a commercial success, they are M. Stanley Whittingham, John B Goodenough and Akira Yoshino. Two have significant British connections and one is the oldest person to receive a Nobel Prize.
The Age of the Battery
With so many portable devices, along with electric vehicles and the growing electricity-storage business demanding lithium batteries, we can say that we are entering the lithium age. The electric battery itself was invented 220 years ago by Alessandro Volta in northern Italy. His voltaic pile, consisting of alternating zinc and silver or copper discs separated by paper or cloth soaked in saltwater, was the first continuous supply of low voltage electricity.
A battery has an anode where an element loses electrons (zinc in the voltaic pile), and a cathode where an element gains electrons. In a battery such as the voltaic pile or the alkaline batteries used today, the chemical reactions that generate the flow of electrons use up the anode and the cathode. The battery runs out of power and cannot be re-used.
When a rechargeable battery is supplied with power the reactants are reformed and then can be used again. For one hundred and sixty years the most widely available rechargeable battery has been the lead-acid battery invented by Gaston Planté. It consists of plates of lead in sulfuric acid. Both the anode and cathode are coated with compounds of lead atoms which can lose or gain electrons. Lead-acid batteries can be recharged and used many times but they are large and heavy and contain highly corrosive acid. They are used in vehicles to start petrol or diesel engines but are of no use for running your mobile phone.
From the beginning of the electronic age, since the Second World War, there has been a demand for small, light, rechargeable batteries. Nickel-cadmium (invented in 1899) and nickel-metal hydride (1989) batteries have been used, particularly in laptop computers. Unfortunately, cadmium is highly poisonous, and the batteries must be quite large to carry sufficient energy.
During the oil crisis of the 1970s when the price of oil rose rapidly and there were fears of a shortage of petrol and other fuels, companies such as Exxon started exploring batteries as alternatives to fossil fuels.
M Stanley Whittingham
Stanley Whittingham was one of the scientists to benefit from Exxon’s interest in batteries. He was born in England in 1941 and attended Stamford School in Lincolnshire. He studied chemistry at Oxford University and completed his doctorate in 1968. Then he made the move to the USA to continue solid state chemistry research at Stanford University. In 1972 he began work for Exxon.
Stanley’s interest was the electrical properties of materials where metal atoms were trapped in the crystal framework of a compound. He found that potassium ions in tantalum disulphide behaved like the cathode of a battery. He began to look into the idea of these materials being used in rechargeable batteries. Tantalum was expensive and heavy, but he discovered that titanium disulfides with lithium ions in the crystal worked as well. He combined this with an anode of lithium metal separated from the cathode by lithium chlorate(VII) in a solvent. The battery worked! It was small, light and provided a lot of electrical energy but there was a problem; it exploded. Whittingham discovered that whiskers of lithium grew between the anode and the cathode, shorting out the battery and catching fire. Nevertheless, the battery did go into production in 1976 and was sold for a time.
Meanwhile, the oil crisis was apparently over and Exxon lost interest in electric power. Stanley left Exxon in 1984 and four years later became a Professor at Binghamton University in New York State where he remains.
John B. Goodenough
It was in the 1970s that John Goodenough became interested in batteries for the same reason as Exxon’s short-lived involvement. By that time, he had already spent over twenty years at the Massachusetts Institute of Technology (MIT). He studied the properties of solid metal oxides which were used in computer memories.
John was born in 1922 in Germany where his father was studying the history of religion. He went to school back home in the USA and studied at Yale University. In 1944 he started his war service and spent five years providing weather reports for the US Army. He returned to his studies and completed his PhD in Chicago in 1952.
MIT would not allow John to shift his research towards batteries so when Oxford University offered him a professorship in 1976, he moved to the UK. With his knowledge of metal oxides, he thought that Whittingham’s lithium battery could be improved. He was right. A cathode made up lithium cobalt oxide could store more energy and supply it at a higher voltage.
In 1986 John returned to the USA to work at the University of Texas. At the age of 97 he is the oldest Nobel Prize winner and he is still doing research.
Akira Yoshino
While oil companies lost interest in batteries, manufacturers of electronic gadgets were still keen to find small, high energy, rechargeable batteries. Japanese companies such as Sony and Toshiba were particularly eager for developments. In 1972 Akira Yoshino began work for the chemical company Asahi Kasei. He was born in 1948 and studied in Osaka and Kyoto, obtaining degrees in petrochemistry and engineering. Asahi Kasei had been producing bulk chemicals like ammonia but had diversified into more valuable compounds.
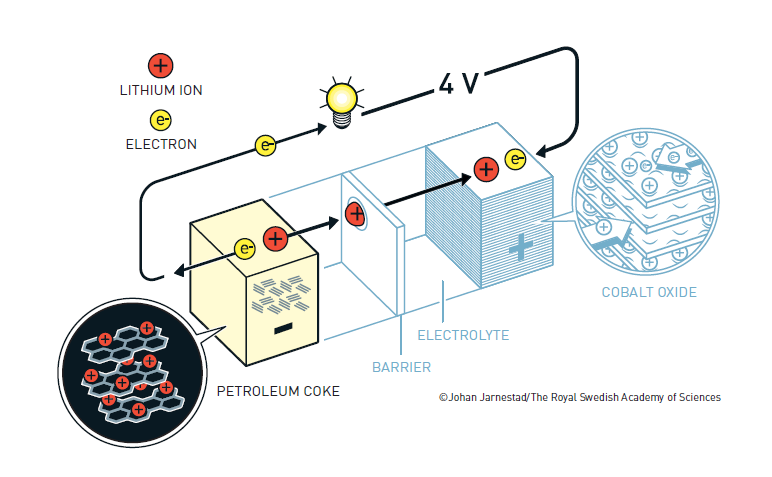
In the early 1980s, Yoshino began work on rechargeable batteries. The designs with lithium metal in the anode were still likely to catch fire during use. Yoshino knew that graphite and other carbon materials could absorb lithium ions. He built a battery with Goodenough’s lithium cobalt oxide cathode and an anode made of petroleum coke. As the battery was charged, lithium ions moved from the cathode through the electrolyte and were embedded in the anode. The battery provided plenty of power, could be recharged many times and was safe; he dropped a large block of iron onto it and it did not burst into flame.
Sony were the first company to use the Asahi Kasei lithium-ion battery designed by Yoshino in 1991. Yoshino continues to work for Asahi Kasei and Meijo University, Nagoya.
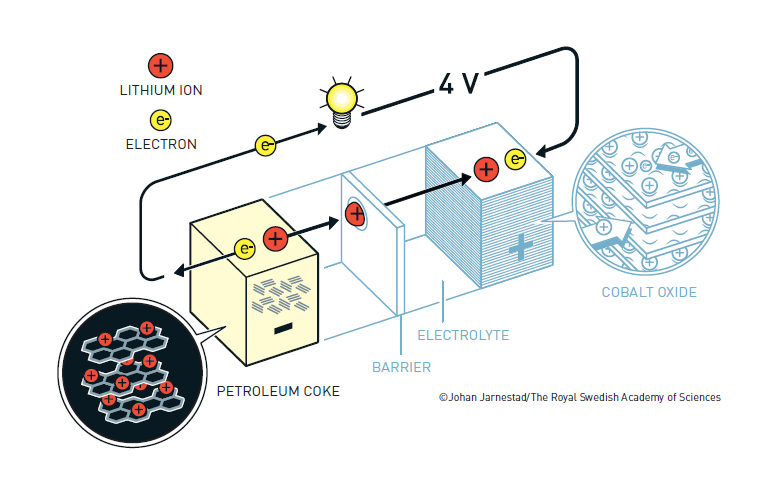
Akiro Yoshino’s design for the lithium-ion battery
The future is lithium, or is it?
Many companies have incorporated lithium-ion batteries into their products. They are vital for the electric car industry being far lighter and having a higher energy content than lead-acid batteries. The batteries have been developed further; John Goodenough found that lithium iron phosphate could replace the cobalt compounds which are toxic. Demand for the batteries and lithium has grown tremendously but there is a problem.
Lithium is an alkali metal like sodium and potassium but is far less common. It was discovered in 1817 (see Freedomtoteach Chemical Anniversaries – 1817: 3 elements) but wasn’t produced on an industrial scale until 1923. It is found in minerals and in seawater but only up to 0.25 parts per million. This makes it expensive and there is doubt that enough of it can be extracted to power all the vehicles (and aircraft?) in the world instead of fossil fuels.
Further discoveries will be needed to bring us fully into the age of the battery but the successes of Whittingham. Goodenough and Yoshino can be celebrated every time you grasp your mobile phone.
Activities
- What advantages does a lithium-ion battery have over a lead-acid battery?
- Whittingham and Goodenough specialise in solid-state chemistry. What does this mean?
- Why are alkali metals such as lithium a good choice for the anode of a battery? Hint. At the anode, atoms lose electrons.
- Why was an oil company like Exxon interested in batteries?
- Give a reason why Stanley Whittingham left Exxon.
- Give a reason why John Goodenough took the job at Oxford University.
- Why was Akiro Yoshino’s discovery that lithium could be trapped in graphite and coke important?
- (a) Make a list of all the devices in your home that use rechargeable batteries (they are very likely to be lithium-ion batteries). (b) Discuss the importance of lithium in your future life.
- M. Stanley Whittingham, John B. Goodenough and Akiro Yoshino are only three of many scientists who researched and developed lithium-ion batteries. Are their achievements worthy of the Nobel Prize? Discuss
Bibliography
You can find out all about the Nobel Prize for Chemistry 2019 on the Nobel Prize website
https://www.nobelprize.org/prizes/chemistry/2019/prize-announcement/
Biographies of the three recipients of the prize are at:
https://en.wikipedia.org/wiki/M._Stanley_Whittingham
https://en.wikipedia.org/wiki/John_B._Goodenough
https://en.wikipedia.org/wiki/Akira_Yoshino
Illustrations
The diagram of the lithium-ion battery is provided by the Nobel Prize committee. They say: The illustrations are free to use for non-commercial purposes. Attribute ”© Johan Jarnestad/The Royal Swedish Academy of Sciences”
Peter Ellis is enjoying life in retirement after a career in chemistry teaching and writing educational materials.