Chemistry Anniversaries – 1864: Before the Periodic Table
The Periodic Table is possibly the most important image in chemistry. It shows how the elements are related to each other and is a sign of the order that chemists have found in the structure of atoms. Mendeleev’s first version of the Table was published in 1869 but here we celebrate the 150th anniversary of the work of two scientists who preceded Mendeleev.
In the 1860s chemistry was changing fast. It was only in 1860 that the majority of chemists settled the argument about the formula of water – was it HO or H2¬O. The decision meant that a list of atomic masses (or weights) could be agreed comparing other elements to hydrogen at 1. New elements were being discovered frequently thanks in part to the new method of spectroscopy (the colour of light emitted by hot atoms) developed by Bunsen and Kirchoff, also in 1860. Many of the new elements were transition or rare earth metals with very similar properties.
Many chemists did not think there was any relationship between elements. Each was unique. Some saw hints of a pattern. One of these was John Newlands. He was born in London in 1837 and studied at the London Royal College of Chemistry. His mother was Italian so in 1860 he set off to fight with Garibaldi for the unification of Italy. Having invaded Naples he returned to London to a career in chemistry. John and his brother formed a consultancy business in 1864 when he developed his ideas about elements.
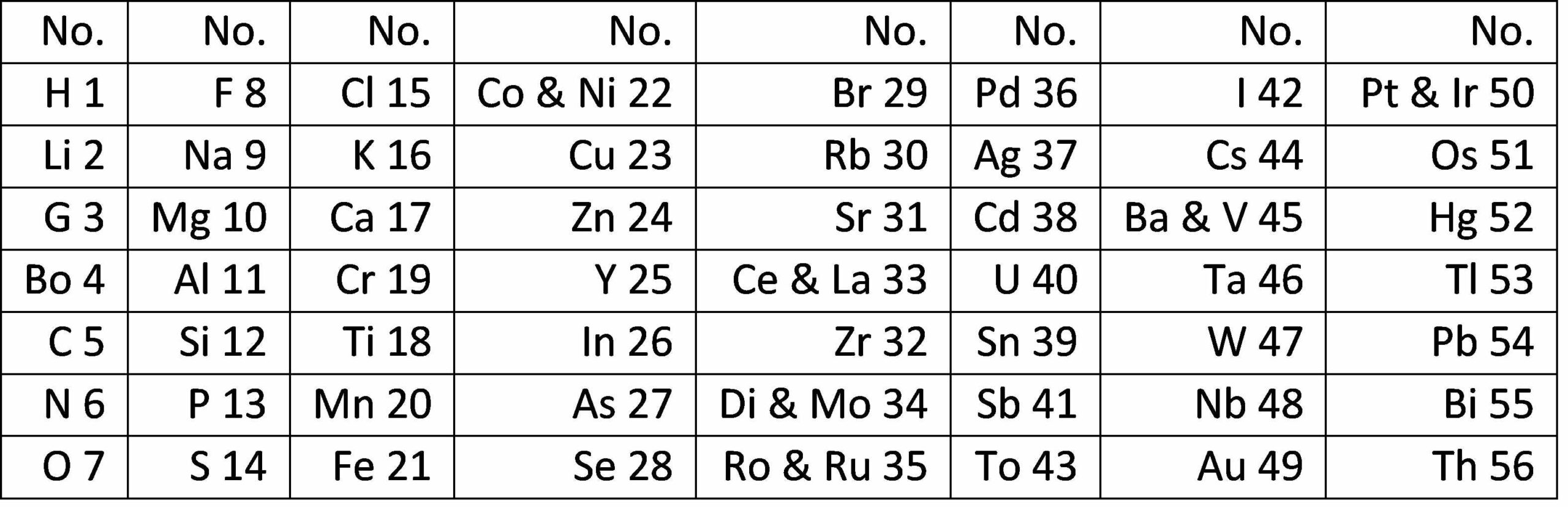
He arranged the 56 known elements in order of their atomic mass. When he looked at the list he noticed that some similar elements had atomic masses that differed by a multiple of 8. For example lithium (7), sodium (23) and potassium (39) differ by 16. He had the idea that the elements were arranged in “octaves” where every eighth element was similar. He drew up a table with seven groups of elements. Newlands’ Law of Octaves was published in Chemical News in 1864. In 1866 he gave a lecture to the Chemical Society in which he compared the elements with the notes of the musical scale. This didn’t go down very well and seemed to the other chemists to be returning to the old days of alchemy as magic. Also there were lots of places in Newlands’ table where the pattern seemed to break down, such as the appearance of the relatively inert copper and silver amongst the far more reactive alkali metals. Newlands’ ideas were rejected and forgotten. He became the chief chemist in a sugar factory.
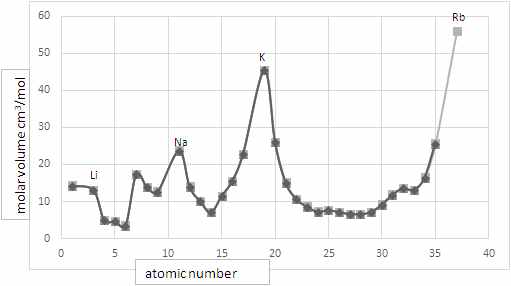
Meanwhile, in Breslau, Germany (now in Poland) Julius Lothar Meyer was writing a textbook of chemistry. Born in 1830 to a medical family he trained in medicine but became interested in chemistry. He studied with Bunsen in Heidelberg before completing his doctorate. Like Newlands, Meyer put the elements in order of atomic mass but he also looked at two other pieces of evidence. The first was a quantity called atomic volume (atomic mass divided by the density of the element in the solid state). When plotted as a graph he noted that similar elements appeared at similar points, for example the alkali metals were at peaks. He thought this supported the idea of “periodicity” i.e. a repeating pattern in the properties of elements.
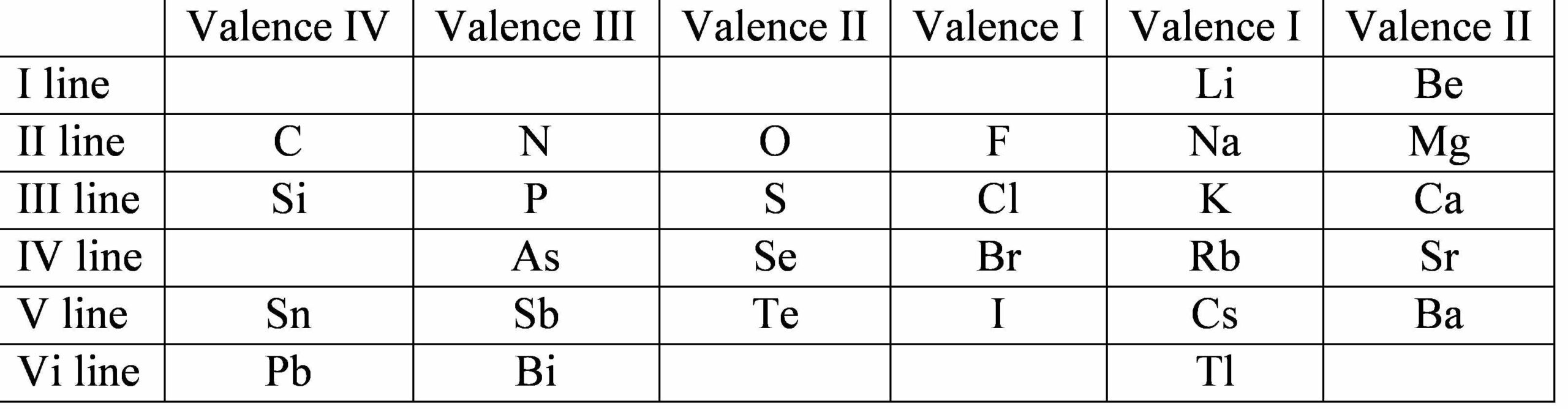
The other factor that Meyer considered was the “valency” of the element. This is the “combining number” of the element with others. For example the valency of the alkali metals and chlorine is 1 so the formulae of the alkali metal chlorides are similar – LiCl, NaCL, KCl etc. Meyer drew up a table with six vertical groups but he only attempted to order 28 elements. His table was included in his book The Modern Theory of Chemistry published in 1864.
Not much notice was taken of Meyer’s ideas until he republished his book in 1870. By this time Mendeleev had announced his Periodic Table. Mendeleev had heard of neither Newlands nor Meyer when he was developing his table in St. Petersburg. Meyer’s atomic volume graph did however become one of the important pieces of evidence that supported Mendeleev’s theory. John Newlands had to wait to 1887 before his part in the story was recognised when he was awarded the Davy Medal by the Royal Society.
Activities
- Find London, Breslau and St. Petersburg on a map. Suggest why Newlands, Lothar Meyer and Mendeleev knew nothing of each other’s work (until after 1869).
- (a) Look at a modern Periodic Table and note the atomic masses of magnesium (Mg), calcium (Ca) and strontium (Sr) in group. (b) Calculate the difference in atomic mass between each pair of elements. (c) Do your results obey Newlands law that the difference is a multiple of 8? (d) Discuss whether there is evidence for or against Newlands Law of Octaves?
- Why do you think that Newlands’s ideas were rejected?
- Why do you think that Lothar Meyer’s work was ignored until after 1870?
- Where do the Alkaline Earth Metals (Be, Mg, Ca, Sr) come in Lothar Meyer’s atomic volume graph? Does this support his law of periodicity?
- Why should Newlands and Lothar Meyer be considered as important figures in the story of the Periodic Table?
Peter Ellis
Bibliography
Oxford Dictionary of Scientists. Pub. OUP
http://www.rsc.org/education/teachers/resources/periodictable/pre16/develop/meyer.htm
http://en.wikipedia.org/wiki/John_Alexander_Reina_Newlands



