The winners of the 2014 Physics and Chemistry Nobel Prizes were announced last week. The discoveries they made show how much the sciences are related and depend on each other. The Physics prize is for the development of the blue light-emitting-diode (LED) and the Chemistry prize is for the invention of microscopes that can see the components of living organisms on the nano-scale. Both involved the basic principles of chemistry – the preparation of new materials with specific properties.
Blue Light
The blue LED was the last to be developed of the trio of red, green and blue needed to produce white light. Red LEDs were developed in the 1950s and were used in the displays of calculators and digital watches from the late 1960s. The green LED followed soon after but the search for the blue LED took until 1992.
An LED consists of a sandwich of semiconductor materials. A semiconductor is a substance that has a fairly high resistance so will only conduct a small current. The elements silicon and germanium in Group 4 of the Periodic Table are semiconductors, but so too are some compounds of the elements on either side such as gallium nitride (GaN). Very pure crystals of these materials are needed. Tiny amounts of other elements are added to the material to produce n- and p-type semiconductors (ones with spare electrons or a shortage of electrons). This is called doping. The sandwich of these materials acts as electronic components such as diodes and transistors. Some emit light when they conduct and these are the LEDs.
Isamu Akasaki began working with gallium nitride in the 1970s. It was not until 1986, when he was working with Hiroshi Amano at Nagoya University in Japan that they produced crystals pure enough to start investigating its semiconductor properties. They then had to discover how to dope the material to produce the different layers of the LED sandwich. They succeeded in producing the blue LED in 1992.

Meanwhile Shuji Nakamura, working at the small chemical Nichia Chemical Corporation in Japan, was making similar progress using slightly different methods. By the mid-1990s both research teams had used their blue LEDS to make a laser. This became the basis for the “blue-ray” DVD players that are now common. Today GaN LEDs are used in backlighting for the screens of mobile phones, tablets, computers and TVs. They are also replacing incandescent and fluorescent lamps as they use very little electricity and have long lifetimes.
85 year old Akasaki is still associated with Nagoya University as is Amano (54). Nakamura (60) is now at the University of California Santa Barbara.
Seeing smaller
In the 17th century, scientists such as Anton van Leeuwenhoek and Robert Hooke developed microscopes to observe objects that could not be seen with the eye alone. In the following centuries lenses and microscopes improved so that smaller and smaller objects could be observed. It was thought, however, that nothing smaller than the wavelength of visible light, about 0.2 micrometres, could be detected. This limit ruled out seeing viruses, the interior of the parts of living cells or individual protein molecules. Electron microscopes can produce images of objects down to a nanometre but they do not work with living organisms. Eric Betzig, Stefan Hell and W.E.Moerner didn’t accept that there was a limit and searched for an alternative way of using light to observe features a few nanometres in size.
The key to their discoveries was fluorescence. When light of one frequency (colour) is shone on a fluorescent molecule it emits light of another frequency. Fluorescent protein molecules can be used like beacons to show the position of things they are attached to. This is like a lighthouse showing the position of a reef or coastline at night and streetlights showing the pattern of roads to passengers in an aircraft. In standard fluorescence microscopy millions of fluorescent molecules are attached to parts of a cell such as the chromosomes. This light given out can be used to form an image, but this doesn’t get over the limit to resolution of 0.2 μm.
Stefan Hell, born in Romania in 1962, was working at the University of Turku, Finland, in the early-1990s. He suggested that a laser beam with a width of a few nanometres could scan across a sample switching the fluorescent molecules on and off. Their beacon light could be recorded building up an image with a resolution far smaller than the limit. In 1995 Hell was offered a position at the Max Planck Institute, Göttingen, Germany, where he was able to develop his STED (Stimulated Emission Depletion) microscope.
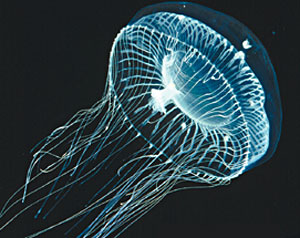
Meanwhile W. E. Moerner (born in 1953) was working on observing the light emitted from a single molecule. He had first achieved this in 1989 working for IBM at San Jose, California. He went on to work with the team using green fluorescent protein (GRP) produced by jellyfish. Using genetic engineering this molecule could be joined to other proteins in various living cells. Moerner found that the light emission could be turned on and off by a laser with a precise frequency allowing him to image the light from individual fluorescent molecules, so long as they were more than 0.2 μm apart.
Eric Betzig, also an American born in 1960, had dreamed of using fluorescent molecules to beat the 0.2 μm limit but had dropped out of scientific research. The discovery of GRPs drew him back to work and he joined the Howard Hughes Medical Institute in Virginia, USA. In 2005 he read about Moerner’s discoveries. He realised that by using fluorescent proteins that gave out light when excited by different frequencies, his dream could become reality. In 2006 he succeeded. A mixture of excitable fluorescent molecules were joined to the proteins making up a structure in a cell. A weak light with a specific frequency set off some of the molecules which were on average more than 0.2 μm apart. An image was taken showing the position of each individual fluorescent molecule. The fluorescence faded and a different batch of molecules were excited. Another image was recorded. This was repeated many times and the images merged. The final picture showed the positions of all the fluorescent molecules revealing the structure down to the nanometer scale.
The work of Hell, Moerner and Betzig has resulted in two different types of nanoscope.
Activities
1 The age of the laureates varies from 85 to 52. How old were the winners when they did the work that earned them the prize?
2 The 2014 chemistry and physics prize winners are American, Japanese and Romanian, but in which countries did they carry out the work that resulted in the prize?
3 What is the meaning of the following terms
(a) Light emitting diode
(b) semiconductor
(c) fluorescence
(d) protein
4 The Nobel Prizes are awarded for discoveries that benefit mankind. What benefits do you think there are in
(a) Blue LEDs;
(b) Nanometre scale light microscopy?
5 Each of the Nobel Prize laureates (winners) had teams of scientists working with and for them. Why do you think the individuals were awarded the prize?
6 The Nobel Prizes were established in 1901 for Physics, Chemistry and Medicine. The work described in this article involved many areas of science. Discuss reasons why it might be time to change the titles of the subjects for which the prizes are awarded.
Bibliography
Details about the Nobel Prizes, the winners and their discoveries can be found on the website
http://www.nobelprize.org/



