John Dalton was born two hundred and fifty years ago. During his lifetime he saw many changes to chemistry, many the result of his own work. His contributions are an important part of school science today.
John was born in the village of Eaglesfield, Cumbria in north-west England, one of seven children. His father was a weaver working at home on a hand-loom. The family were members of the religious group, the Quakers, so John attended the local Quaker school. He was a quick learner but when he was 12 the teacher retired. John took over the tiny school, teaching pupils both younger and older than himself. In 1781 John was invited to join a brother teaching at a larger Quaker school in Kendal. Being a school master did not bring in a lot of money but they were expected to maintain an appearance of being above the working class. During this period John was tutored by educated gentlemen who had settled in the area. They encouraged John’s growing interest in science, particularly the study of the weather.
The universities of Oxford and Cambridge were closed to people like John who were not members of the Church of England. In the seventeenth century other colleges opened to educate “non-conformists”. In 1793, on the recommendation of one of his tutors, John Dalton was offered a lecturer’s post at the New College in Manchester. His duties were to teach mathematics and natural philosophy (i.e. physics) and chemistry, for a salary of £80 a year minus £27.50 for his college room and food. He quickly joined the Manchester Literary and Philosophical Society and became one of the leading members.
John lived in Manchester for the rest of his life but in 1800 he opened his own Mathematical Academy and earned a living educating “young gentlemen”. He carried out his own research and gave public lectures on the many subjects that interested him.
John’s first published work was a study of colour blindness. He had a severe form of it, meaning that he could not distinguish between reds, greens or blues. He once bought a pair of bright red stockings for his mother thinking they were her usual dark blue. His paper became so popular that the disability was re-named Daltonism.
Much of John’s research was concerned with the atmosphere. He made many meteorological measurements but also carried out experiments to test his ideas about gases. This lead him to theories of heat and pressure and to his atomic theory.
When John was born in 1766 most chemists still considered “air” to be a single substance although fixed air and flammable air were known (see FreedomtoTeach: 1766 – Three Papers by Henry Cavendish, Jan 2016) and they thought that when substances burned they gave off a substance known as phlogiston. By 1803 the work of the French scientist Antoine Lavoisier had begun a revolution in chemistry. There were now about thirty known elements including the gases that made up most of the air, oxygen and nitrogen. It was also known that many substances were compounds of two or more elements such as water (hydrogen and oxygen) and carbon dioxide (carbon and oxygen). Many scientists thought that substances were made up of tiny particles called atoms but there was little agreement about their properties.
John Dalton carried out experiments on dissolving various gases in water and other liquids and on mixtures of gases. In 1803 he published his discoveries. He said that in a mixture, different gases behaved as if they filled the space alone. This lead him to propose his Law of Partial Pressures (The total pressure of a mixture of gases is the sum of the pressures each gas would exert if it occupied the volume alone). He also realised that many of the physical properties of a gas were due to the weight (or mass, we say today) of the particles of which it is made up. Over the next few years he developed his ideas and friends pushed him to reveal them. In 1808 his book “A New System of Chemical Philosophy” was published. Much of the book is concerned with heat and the properties of gases but in the last section he introduces his Atomic Theory. Dalton was very interested in the mass of each element that combined to make compounds and this allowed him to draw up the first table of atomic masses. From his book we can pick out the main points he made about atoms.
- All matter is made of atoms;
- Atoms cannot be broken up, created or destroyed;
- All the atoms of an element have the same size, mass and other properties but are different to atoms of another element;
- Atoms of different elements join to form compounds in simple ratios (1:1, 1:2, etc.).
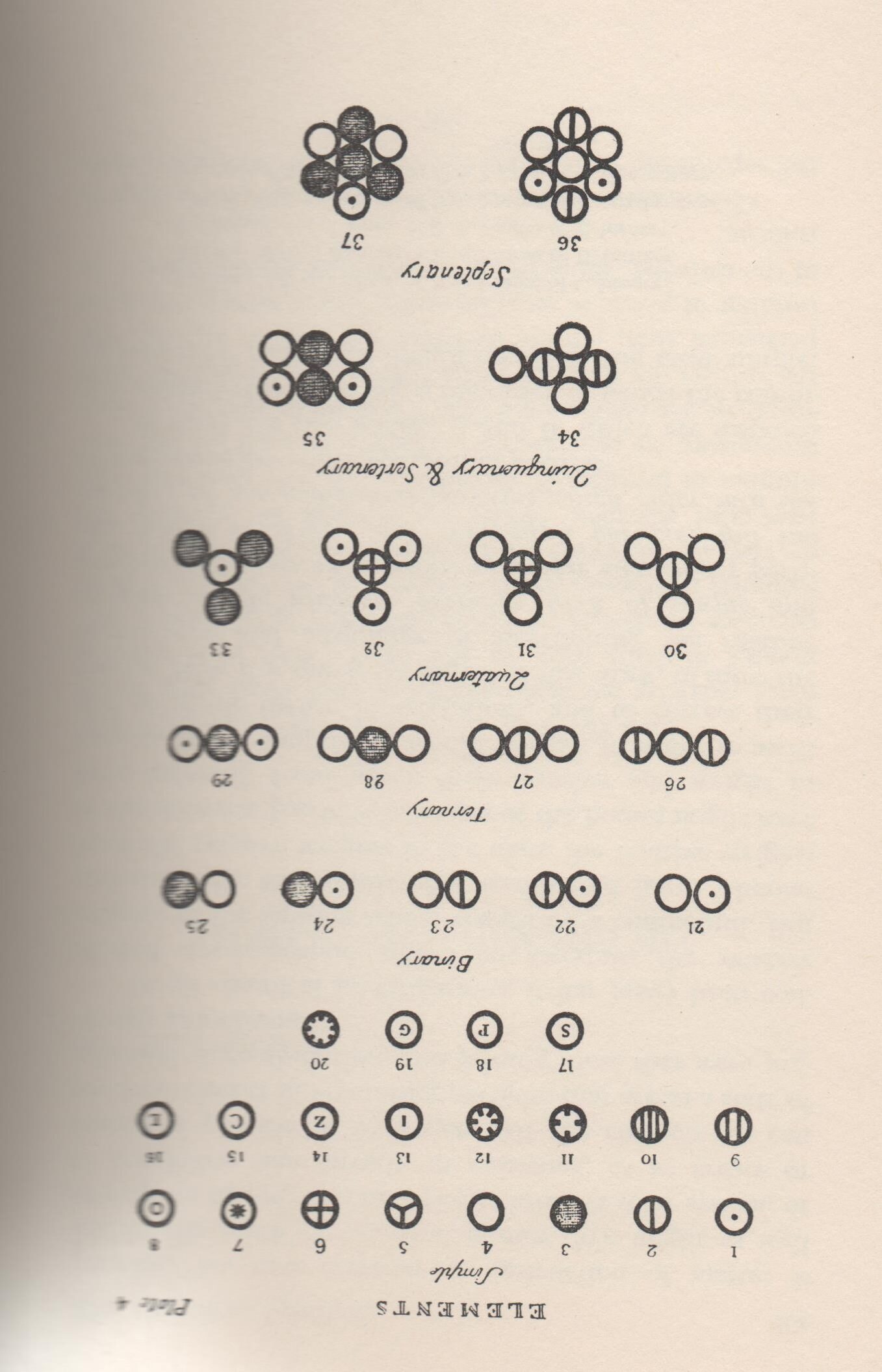
Dalton thought that the simplest compound of two elements would consist of one atom of each element joined together (a “compound-atom”). He was certain that water, being the simplest compound of hydrogen and oxygen, had compound-atoms or molecules made up of 1 atom of each. He drew symbols of elements to show the compounds that could be made.
Dalton’s book became popular amongst scientists and he was soon receiving honours. In 1810 he refused to join the Royal Society because of the cost of membership but later he became a Fellow when supporters offered to pay. He was not well off until late in his life. The University of Oxford gave him two honorary degrees and he had an audience with King William IV. He continued to teach in Manchester but in 1833 he was given a state pension on the recommendation of Charles Babbage, the computing pioneer.
Pictures show him as bespectacled man. He was stubborn, refusing to accept that some of his ideas may be wrong, and a little eccentric. He took his “weekend” break mid-week and did not get married because, so he said, “he never got round to it”. He became ill in his last few years and died in 1844. Although he was still a Quaker and expecting a quiet funeral, the city of Manchester organised a lavish ceremony with four hundred policemen controlling the 40,000 people that lined the route of his coffin. Statues were erected to him and, not far from his birthplace, the Church of England built his official memorial church – a strange way of remembering a Quaker.
John Dalton’s work on gases and his atomic theory spurred other scientists to continue the building of a new chemistry. Over the following years, however, much of his work was corrected. His original list of atomic masses was inaccurate. This was partly because of the simple apparatus he had available and partly because he thought the formula of water was HO and so calculated that oxygen had an atomic mass of 8 (early lists had it as 7). His symbols dropped out of use because the letters-based system of Swedish chemist, Berzelius, was simpler to remember and to print. In the 1860s it was proved that water was in fact H2O. This meant that he was wrong to say that the simplest compound between two elements was always one atom of each.
At the turn of the twentieth century, discoveries in radioactivity and with cathode rays showed that atoms were not indestructible and had a structure made up of even tinier particles. Atoms of one element could turn into atoms of another and, in nuclear fission, they could be split apart. The discovery of isotopes showed that the atoms of an element were not all identical.
It looks therefore as though all of John Dalton’s Atomic Theory has in fact proved to be wrong. Nevertheless, his work is still looked on as one of the foundations of modern chemistry which was built on by later chemists. This is rather like the way the laws of Isaac Newton have been modified and corrected by Albert Einstein’s Theory of Relativity. So on this 250th anniversary of his birth let us celebrate the life and work of John Dalton.
Tasks.
1) John Dalton’s first scientific interest was in the weather. He made his own barometers and thermometers.
- Keep a diary of the weather where you live for a week.
- Explain why weather-watching is a good place to start doing science.
2) Imagine you are John Dalton, working as a teacher at the age of twelve. Describe the challenges and pleasures this might have brought him.
3) Look at the table of symbols.
- What compounds are represented by the formulas numbered 24 and 27?
- Use Dalton’s symbols to draw molecules of water (H2O) and methane (CH4).
4) Explain why Dalton’s atomic symbols were replaced by the system of letters we use today.
5) Dalton thought of atoms as hard, unbreakable balls. Explain how modern ideas of atoms are different.
6) It seems that all of Dalton’s Atomic Theory was wrong. Why is John Dalton still celebrated as a great scientist?
Bibliography
John Dalton, by Hugh Turner, pub. The Printing House, Cockermouth
A New System of Chemical Philosophy, John Dalton (edition published by Peter Owen Ltd., London 1965)
Illustrations
Dalton’s symbols taken from the 1965 edition of his book (see above) but copied from his original manuscript.
Key: Simple atoms (i.e. elements) as numbered in the picture in order of atomic weight (* are now known to be compounds).
| 1 | Hydrogen | 11 | Strontites * |
| 2 | Nitrogen | 12 | Barytes * |
| 3 | Carbon | 13 | Iron |
| 4 | Oxygen | 14 | Zinc |
| 5 | Phosphorus | 15 | Copper |
| 6 | Sulfur | 16 | Lead |
| 7 | Magnesia * | 17 | Silver |
| 8 | Lime * | 18 | Platinum |
| 9 | Soda * | 19 | Gold |
| 10 | Potash * | 20 | mercury |